Learning Outcomes
i. Master the fundamental rules for assigning oxidation numbers to free elements, ions, molecules, and atoms.
ii. Accurately determine oxidation numbers in ionic and covalent compounds, including simple and complex ions.
iii. Utilize oxidation numbers to identify oxidizing and reducing agents in redox reactions.
iv. Apply oxidation numbers to balance redox equations, ensuring a balanced representation of electron transfers.
Introduction
In the intricate realm of chemistry, where atoms engage in a silent dance of electron exchange, oxidation numbers emerge as a crucial tool, revealing the electron distribution and gain or loss by individual atoms. This lesson will unveil the rules governing oxidation number assignment, empowering you to navigate the intricate world of redox processes with precision and clarity.
Rule 1: Free Elements Exhibit Zero Oxidation Number
Elements in their free state or uncombined form, such as sodium (Na), oxygen (O2), and sulfur (S8), possess an oxidation number of zero. This reflects their neutral state, with no electron gain or loss.
Rule 2: Ionic Compounds Adhere to Charge-Based Oxidation Numbers
In ionic compounds, the oxidation state of an element is equal to its charge. For instance, in sodium chloride (NaCl), sodium ions (Na+) have an oxidation number of +1, while chloride ions (Cl-) have an oxidation number of -1.
Rule 3: Covalent Compounds Follow Electron Distribution Principles
In covalent compounds, oxidation numbers are determined by the electron distribution around the atoms. More electronegative atoms tend to attract electrons, gaining a negative oxidation number, while less electronegative atoms lose electrons, acquiring a positive oxidation number.
Examples of Oxidation Number Assignment in Covalent Compounds:
Water (H2O): Hydrogen (H) has an oxidation number of +1, while oxygen (O) has an oxidation number of -2.
Methane (CH4): Carbon (C) has an oxidation number of -4, while hydrogen (H) has an oxidation number of +1.
Rule 4: Oxidation States in Complex Ions
In complex ions, the oxidation state of the central metal ion is determined by the charges of the ligands and the overall charge of the complex ion.
Example of Oxidation Number Assignment in a Complex Ion:
Iron(III) chloride (FeCl3): Iron (Fe) has an oxidation number of +3, while chloride (Cl-) has an oxidation number of -1.
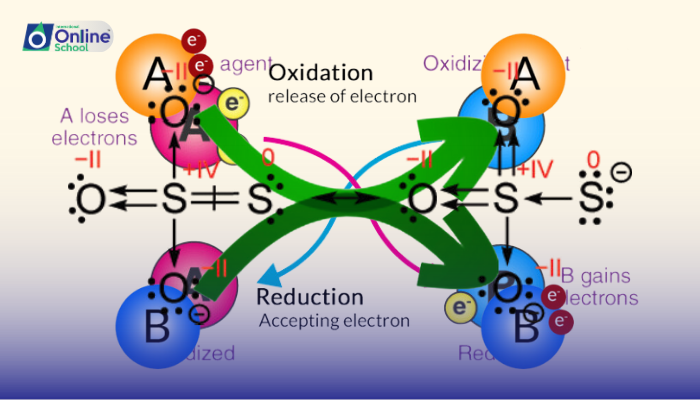
i. Significance of Oxidation Numbers: Unveiling Electron Flow
Oxidation numbers play a pivotal role in identifying oxidizing and reducing agents in redox reactions. The species that loses electrons and increases its oxidation number undergoes oxidation and acts as the reducing agent, while the species that gains electrons and decreases its oxidation state undergoes reduction and acts as the oxidizing agent.
ii. Balancing Redox Equations: A Matter of Electron Accounting
Balancing redox equations requires a careful accounting of electron transfers. By ensuring that the number of electrons lost by the reducing agent equals the number gained by the oxidizing agent and that the sum of oxidation numbers on both sides of the equation remains equal, we achieve a balanced representation of the electron exchange process.
Oxidation numbers, the language of electron distribution and transfer, provide a powerful tool for understanding redox processes. By mastering the rules of oxidation number assignment, we gain insights into the electron dynamics of chemical reactions, enabling us to identify oxidizing and reducing agents, balance redox equations, and appreciate the intricacies of electron flow in the grand symphony of chemistry.